By Dr. Matthew Leech, post-doctoral fellow, University of Greenwich
According to the International Energy Agency (IEA), global energy-related carbon dioxide emissions were estimated to be approximately 33 gigatonnes (Gt).1 It is perhaps unsurprising that the two largest sources of carbon dioxide emissions arise from transportation and the generation of electricity (Figure 1). However, what is surprising is that the aggregate global emissions of the pharmaceutical sector far outweigh those of the automotive industry – 52 million tonnes vs 46.4 million tonnes in the year 2018.1

Figure 1: World carbon dioxide emissions by sector between 1990 and 2010. Source – https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions
This is because a large proportion of chemical syntheses in an industrial setting are conducted under thermochemical conditions, meaning that they must be heated to high temperatures for prolonged periods of time. These conditions are very energy intensive, and the reagents used are often hazardous and difficult to remove from the final product. The latter is particularly problematic for redox reactions (reactions involving the addition or removal of electrons), where toxic metal complexes are often used.
In contrast, the electrochemical synthesis of organic molecules, also known as Organic Electrosynthesis, uses the cheapest and most versatile redox agent available on the market, electricity, to perform chemical transformations at room temperature. Organic electrosynthesis has experienced a resurgence in interest in recent years, owing to the increasing availability of affordable and standardised equipment,2 which has improved experiment reproducibility, and the growing desire by the chemistry community to find milder and greener reaction conditions. A typical modern academic electrosynthesis setup can be seen in Figure 2.

Figure 2: Modern organic electrosynthesis equipment, comprising of a combined stirrer/potentiostat, cell lid, 10 mL glass cell, and two carbon graphite electrodes.
Aside from saving energy by switching from thermochemical to electrochemical synthesis, electrosynthesis is often safer than traditional methods, as it reduces or eliminates the need for toxic solvents and catalysts. In 2019 it was reported that substituted lactones, which are known for their fungicidal, antibiotic, and anticancer properties, can be synthesised using photochemistry (Scheme 1).3 However, the reported procedure necessitates the use of a potentially toxic nickel catalyst, in addition to a solvent mixture comprising of benzene and 1,4-dioxane, both of which are suspected carcinogens. Fortunately, a complimentary electrochemical method was reported in the same year which not only removed the need for the nickel catalyst, but also uses methanol as a solvent, which is less toxic and is considered to be greener by comparison (Scheme 1).4
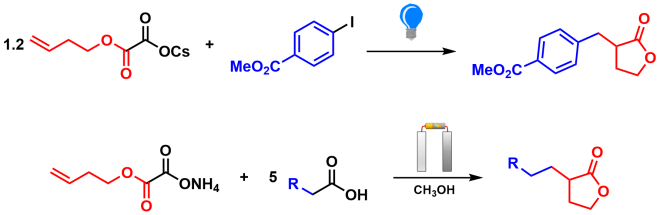
Scheme 1: Synthesis of substituted γ-butyrolactones via photochemical (top) and electrochemical (bottom) methods.
Photochemistry also necessitates the use of a photocatalyst, a compound which can convert absorbed light into useable electrons. These are normally based upon third-row transition metals such as iridium, which makes them very expensive (a typical photocatalyst costs between £40 – 60 for 0.1 g), which usually limits the uptake of photochemical methods within industry. In contrast, electricity is cheap, with 1 mole of electrons (6.02×1023 electrons) costing around £0.83.4
Furthermore, through electrosynthesis, it is possible to avoid the use of reactive and often highly toxic reagents by generating them in-situ. For example, methoxymethyl (MOM) ethers, which are used as protecting groups in organic chemistry, are normally synthesised using chloromethyl methyl ether (CMME) which, aside from being flammable and toxic, is known to cause cancer. However, it is now possible to synthesise these ethers using electrosynthesis at room temperature, using a very low current, inexpensive graphite electrodes, and greener solvents (Scheme 2).5,6

Scheme 2: Electrochemical synthesis of methoxymethyl ethers from carboxylic acid derivatives in methanol at room temperature using graphite electrodes.
But organic electrosynthesis is not limited to university laboratories, as electrosynthetic methodologies can often be scaled-up to be used in industrial laboratories with relative ease. Perhaps the most well-known example of industrial scale electrosynthesis is the electrohydrodimerisation of acrylonitrile into adiponitrile by Monsanto, which is a key intermediate in the manufacturing of nylon (Scheme 3).7–9 Under aqueous conditions, it has been possible to synthesise approximately 300 000 tons of adiponitrile worldwide, with the sole by-product being oxygen, arising from the oxidation of water.

Scheme 3: Industrial electrochemical synthesis of adiponitrile from two acrylonitrile molecules using cadmium and stainless-steel electrodes under aqueous conditions.
In summary, organic electrosynthesis represents a modern, greener, more economical, and safer alternative to traditional chemical syntheses. With the advent of new and more user-friendly equipment, the field has experienced a resurgence in interest, with many new methodologies being developed in recent years. Through the use of electrosynthesis, the challenge of making the pharmaceutical sector greener is starting to look much more achievable.
References
1 L. Belkhir and A. Elmeligi, Carbon footprint of the global pharmaceutical industry and relative impact of its major players, J. Clean. Prod., 2019, 214, 185–194.
2 M. Yan, Y. Kawamata and P. S. Baran, Synthetic Organic Electrochemistry: Calling All Engineers, Angew. Chem. Int. Ed., 2018, 57, 4149–4155.
3 L. E. Overman, N. A. Weires and Y. Slutskyy, Facile Preparation of Spirolactones by an Alkoxycarbonyl Radical Cyclization Cross-Coupling Cascade, Angew. Chem. Int. Ed., 2019, 58, 8561–8565.
4 A. Petti, M. C. Leech, A. D. Garcia, I. C. A. Goodall, A. P. Dobbs and K. Lam, Economical, Green and Safe Route Towards Substituted Lactones via the Anodic Generation of Oxycarbonyl Radicals, Angew. Chem. Int. Ed., 2019, 58, 16115–16118.
5 X. Luo, X. Ma, F. Lebreux, I. E. Markó and K. Lam, Electrochemical methoxymethylation of alcohols – A new, green and safe approach for the preparation of MOM ethers and other acetals, Chem. Commun., 2018, 54, 9969–9972.
6 C. G. W. van Melis, M. R. Penny, A. D. Garcia, A. Petti, A. P. Dobbs, S. T. Hilton and K. Lam, Supporting-Electrolyte-Free Electrochemical Methoxymethylation of Alcohols Using a 3D-Printed Electrosynthesis Continuous Flow Cell System, ChemElectroChem, 2019, 6, 4144–4148.
7 D. E. Danly, Development and Commercialization of the Monsanto Electrochemical Adiponitrile Process, J. Electrochem. Soc., 1984, 131, 435C.
8 M. M. Baizer, Electrolytic Reductive Coupling, J. Electrochem. Soc., 1964, 111, 215.
9 M. C. Leech, A. D. Garcia, A. Petti, A. P. Dobbs and K. Lam, Organic electrosynthesis: from academia to industry, React. Chem. Eng., 2020, Advanced Article, DOI: 10.1039/D0RE00064G
