By Annabelle Wong, Member-at-Large for the GCI
With heightened concerns on greenhouse gas (GHG) emissions in recent years, scientists and engineers have come up with some innovative solutions to mitigate carbon dioxide emissions. One solution is to utilize and covert CO2 to everyday products such as fuels and plastics. Recently I learned that CO2 is now being converted into cement on an industrial scale.
Concrete is the most common construction material for buildings, roads, and bridges. Cement is one of the components of concrete and acts as a glue to hold concrete together. However, cement manufacturing is an energy-intensive process and the cement/concrete industry is one of the biggest CO2 emitters. In fact, 5% of the global GHG emission stems from cement production.1–3 To understand why so much CO2 is released, let’s first take a look at how cement is produced.
To make cement, limestone (calcium carbonate, CaCO3), silica (SiO2), clay (containing mostly Al2O3), and water are mixed and heated. This reaction produces a significant amount of CO2 and is called calcination. During calcination, at temperatures above 700 °C, limestone is decomposed to lime, or calcium oxide, and CO2 (Reaction 1). Then, lime reacts with SiO2 to form calcium silicates (C2S in simplified cement chemist notation, where C = CaO, S = SiO2) and tricalcium silicates (C3S) as the temperature ramps up to 1500 °C (Figure 1). The final product, called clinker, is then cooled and milled into a fine power. Afterwards, minerals such as gypsum (CaSO4) are added to make cement.4 A useful animation of cement making can be found here.5
CaCO3 (s) → CaO (s) + CO2↑ (g) (1)
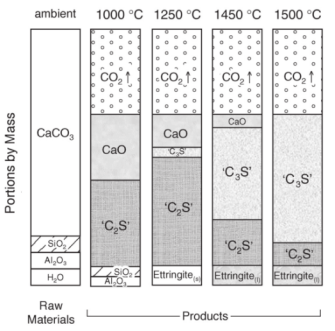
Figure 1. Raw materials are heated up to 1500 degrees C to synthesize clinker. The ratios of products yielded at various temperatures are shown. [4]
The idea of rendering the cement process more sustainable is to capture CO2 from a cement plant’s flue gas and convert it to the starting material of cement, CaCO3, creating a carbon neutral process. Scientists and engineers have been developing different technologies to achieve this goal. For example, at Calera, a company in California, CO2 is first converted to carbonic acid. Then, Ca(OH)2, which can be found in industrial waste streams, is added to convert carbonic acid to CaCO3 and water. The overall reaction is shown in Reaction 2.8
CO2 + Ca(OH)2 → CaCO3 +H2O (2)
Iizuka et al.9 suggested that the Ca(OH)2 and calcium silicates can be extracted from waste concrete, such as concrete from dismantled buildings, as a source of calcium ions. Their methodology is similar to Calera’s, but the carbonic acid is used for the extraction of calcium ions from waste cement (Figure 2).9 Furthermore, Vance et al. has shown that liquid and supercritical CO2 can accelerate the formation of CaCO3 from Ca(OH)2.1
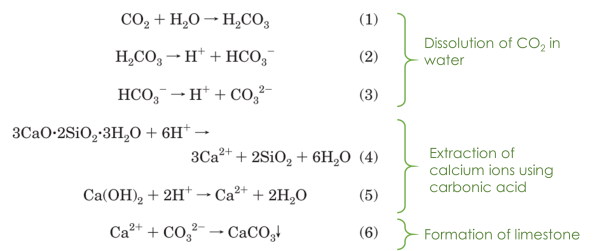
Figure 2. Recycling CO2 and concrete to make limestone, the starting material of cement. [9]
As mentioned above, fuel combustion and use of electricity also contribute to the CO2 emissions of cement production. In this way, other efforts to reduce CO2 emissions include recovering heat from the cooled clinker,5 utilization of alternative fuels, reduction of clinker in cement,3,11 and utilization of cement to absorb CO2.2
With innovative research, development, and commercialization of CO2 conversion technologies, I am optimistic that they will have a solid impact in the near future at the global scale. However, despite the current advances in CO2 conversion technology, a collaborative effort on both CO2 capture and utilization, along with the infrastructure to bridge these two technologies together, is essential to realize a carbon- neutral society.
References
(1) Vance, K.; Falzone, G.; Pignatelli, I.; Bauchy, M.; Balonis, M.; Sant, G. 2015.
(2) Torrice, B. M. Chemical and Engineering News. November 2016, p 8.
(3) Crow, J. M. Chemistry World. 2008.
(4) Maclaren, D. C.; White, M. A. J. Chem. Educ. 2003, 80 (6), 623–635.
(5) Cement Making Process http://www2.cement.org/basics/images/flashtour.html.
(6) Explore Cement http://www.wbcsdcement.org/index.php/about-csi/explore-cement?showall=&start=2.
(7) Mason, S. UCLA scientists confirm: New technique could make cement manufacturing carbon-neutral http://newsroom.ucla.edu/releases/ucla-scientists-confirm:-new-technique-could-make-cement-manufacturing-carbon-neutral.
(8) The Process http://www.calera.com/beneficial-reuse-of-co2/process.html.
(9) Iizuka, A.; Fujii, M.; Yamasaki, A.; Yanagisawa, Y. Ind. Eng. Chem. Res. 2004, 43, 7880–7887.
(10) Technology http://carboncure.com/technology/.
(11) Cement Industry Energy and CO2 Performance: Getting the Numbers Right (GNR); 2016.

