By Diya Zhu, Member-at-Large for the GCI
A carbonyl functionality is a functional group composed of a carbon atom double-bonded to an oxygen atom (C=O). It is ubiquitous in nature as well as widely employed and studied in all areas of chemistry. In this blog, we will explore two common synthetic processes involving carbonyl groups with greener alternative reagents.
Dethioacetalization with NH4I
Carbonyl-containing compounds are abundant in nature, expressing a wide range of functionality. As targeted in many natural and non-natural product syntheses, the protection and deprotection of the carbonyl functional groups are critical and often require multiple steps. Common carbonyl protecting groups are dithianes and dithiolanes due to their easy accessibility and high stability under acidic/basic conditions. The traditional dethioacetalization is generally performed utilizing heavy-metal salts such as mercury(II) chloride, silver(II) nitrite, ceric ammonium nitrate, and selenium dioxide, of which the resulting waste is very toxic to the environment.1
From 1989 to 2005, serval hypervalent iodine compounds such as bis(trifluoroacetoxy)-iodobenzene (BTI), Dess-Martin periodinane (DMP), and o-iodoxybenzoic acid (IBX) have been employed as dethioacetalization reagents due to their low toxicity, high selectivity, and metal-ion free nature. While these reagents have a smaller environmental impact, they are still required in excess amount, which is economically wasteful.2, 3
Finally, in 2011, Ganguly and Mondal reported a mild, efficient, and greener dethioacetalization protocol using a catalytic amount of ammonium iodide with hydrogen peroxide.3 In this work, the deprotection was carried out with 10 mol% of nontoxic ammonium iodide and 30% hydrogen peroxide as the terminal oxidizer in an aqueous medium in the presence of sodium dodecylsulfate (SDS). This protocol (Figure 1) shows a high yield (>90%) deprotection of 1,3–dithianes and dithiolanes of activated aromatics and even deactivated and sterically encumbered substrates. The high tolerance, low environmental impact, mildness, operational simplicity, high throughput, and generality of the protocol make it an intriguing alternative.

The greener dethioacetalization protocol by Ganguly and Mondal. [3]
Various catalyst systems for the reduction of carbonyl compounds have been established, such as Meerwein–Ponndorf–Verley (MPV) reduction.4 However, only a handful of protocols were reported for the transfer hydrogenation of aldehydes due to the difficulty in controlling the chemoselectivity in the process.
In these conversional protocols of transfer hydrogenation, many side-reactions (for example, aldol condensations) take place after deprotection by the base. The heavy-metal catalysts (such as rhodium, iridium, and ruthenium complexes) are expensive and often poisoned by the substrates, resulting in non-recyclable catalysts and many side-products. In addition, the hydrogenation of carbon-carbon double bonds (C=C) and aldehydes compete, resulting in poor chemoselectivity.5,6 Due to these drawbacks, there was a significant desire for more efficient and environmentally benign catalytic systems.
In the last decade, iron catalysts have received much attention due to their nontoxic, abundant, and inexpensive qualities. In 2013, Beller and his colleagues published an efficient iron-based catalyst system for the highly selective transfer hydrogenation of aldehydes under mild conditions.6 In this system, they suggested that iron-tetraphos complexes [(Fe(BF4)•6H2O and P(CH2CH2PPh2)3) are able to catalyze a wide range of substrates such as aromatic, aliphatic, and α,β-unsaturated aldehydes to the corresponding alcohols in excellent yields (>99%). Without the presence of a base, formic acid is used as a cheap, environmental friendly, and easy to handle hydrogen source. In addition, no significant amounts of side products were observed.
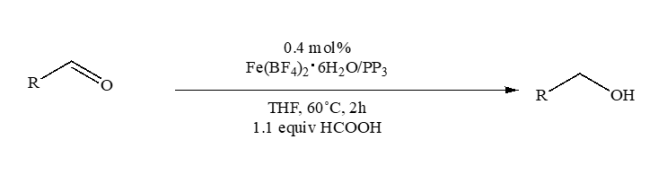
The iron-catalyzed transfer hydrogenation with formic acid. [6]
References:
- J. Corey, B. W. Erickson, Journal of Organic Chemistry 36 (1971), 3553; E. Vedejs, P. L. Fuches, Journal of Organic Chemistry 36 (1971), 366.
- S. Kirshnaveni, K. Surendra, Y. V. D. Nageswar, K. R. Rao, Synthesis 15 (2003), 2295. DOI: 10.1055/s-2003-41055
- C. Ganguly, P. Mondal, Synthetic Communications 41 (2011), 2374. DOI: 10.1080/00397911.2010.502995
- Gladiali, E. Alberico, Chemistry Society Reviews 35 (2006) 226. DOI: 10.1039/B513396C
- S. M. Samec, J.-E. Bäckvall, P. G. Andersson, P. Brandt, Chemistry Society Reviews 35 (2006), 237. DOI: 10.1039/b515269k
- Wienhöfer, F. A. Westerhaus, K. Junge, M. Beller, Journal of Organometallic Chemistry 744 (2013) 156. DOI: 10.1016/j.jorganchem.2013.06.010
- Sigma Aldrich Alternative Product Page. http://www.sigmaaldrich.com/chemistry/chemistry-products.html?TablePage=119262253 (accessed Oct 15, 2017).
