By Cici Chenyu Yue, Undergraduate student, Member-at-Large for the GCI
Given the urgent goal to reach net zero by 2050, many new carbon capture techniques have emerged in literature. One such promising method is Monoethanolamine (MEA) amine scrubbing due to its high capacity and usefulness at industrially relevant scales. In MEA amine scrubbing, MEA reacts with CO2 in the atmosphere to generate carbamate (RNHCOO–) and bicarbonate (HCO3–), two which are economically useful reagents. To return back to MEA, the carbamate must then go through a regeneration step in water, whereby the carbamate N-C bond is broken and CO2 is released. Unfortunately, this step requires high temperatures (110-130 °C) since the N-C bond is relatively strong. The formation of additional CO2 from this energy intensive step makes it key to lower the regeneration temperature for future industrial scale application.
One approach to temperature reduction is through solid acid catalysts (SACs); acidified metal compounds that accelerate the rate of CO2 desorption via proton or electron transfer. While these catalysts drastically lower the activation energy barrier, their effectiveness is limited by their poor surface area and porosity. To solve these issues, the authors of a recent work in Environmental Science & Technology took inspiration from metal organic frameworks (MOFs), materials that are renowned for their high surface area, and propose two-dimensional cobalt−nitrogen-doped carbon nanoflakes (Co–N–C NF).
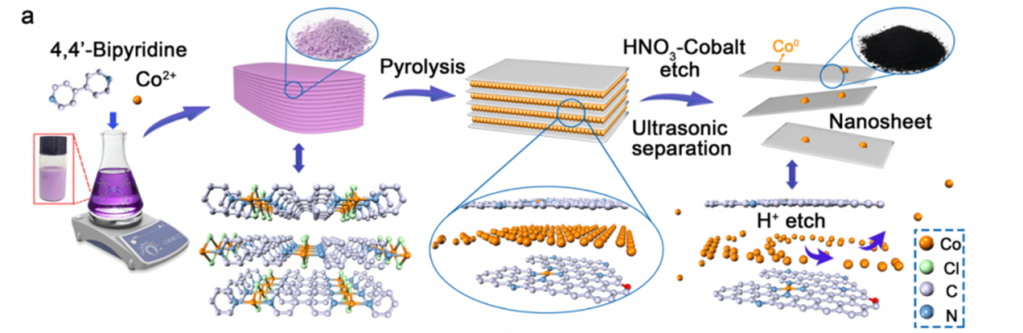
To synthesize these materials, the authors first created a bulk MOF before pyrolyzing and exfoliating the material into a 2D sheet. Even after pyrolysis, Co–N–C NF retains the porosity of the original MOF material, resulting in high hydrophilicity and surface area. Additionally, strong cobalt-nitrogen bonding interactions within Co–N–C NF prevent MEA amino groups from stripping away the catalyst surface, which improves stability over many cycles. When incorporated into MEA carbon capture systems, Co–N–C NF was able to increase the overall CO2 absorption efficiency by 28%. Furthermore, during regeneration, the CO2 desorption rate was 281% higher than the noncatalytic process at 88°C. This allows Co–N–C NF to operate optimally under considerably lower temperatures than other counterparts, thus drastically improving its energy efficiency.
This innovation in MEA carbon capture represents one of the many major steps that materials chemists have taken in the field of green chemistry. If this work was inspiring, be sure to check out our other blog posts and our website for more green chemistry content.
References:
Colin, Baird. Environmental Chemistry, 5th Edition. (Macmillan Higher Education), 2012.
Lei Xing, Meng Li, Mingyue Li, Teng Xu, Yuchen Li, Tieyue Qi, Huanxin Li, Zhigang Hu, Guang-ping Hao, Shihan Zhang, Tony David James, Boyang Mao, and Lidong Wang. MOF-Derived Robust and Synergetic Acid Sites Inducing C–N Bond Disruption for Energy-Efficient CO2 Desorption. Environmental Science & Technology 2022 56 (24), 17936-17945. DOI: 10.1021/acs.est.2c06842
Meys, R.; Kätelhön, A.; Bachmann, M.; Winte, B.; Zibunas, C.; Suh, S.; Bardow, A. Achieving net-zero greenhouse gas emission plastics by a circular carbon economy. Science 2021, 374, 71– 76, DOI: 10.1126/science.abg9853
