By Eloi Grignon, PhD student in the Seferos Group at the University of Toronto
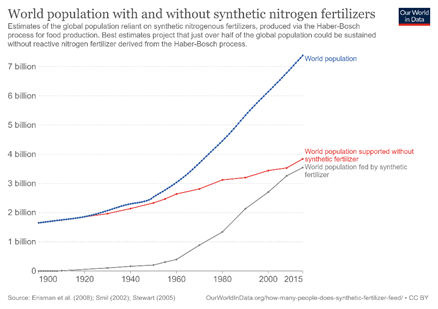
Modern agriculture does not begin in a field but in a reactor. Here, nitrogen and hydrogen enter and are transformed to ammonia by iron catalysts on the reactor bed. This ammonia, which is produced at the colossal scale of 230 million tonnes per year, is the fertilizer that sustains the nearly 8 billion people on our planet. Without it, our population would drop by half.1
This awesome industrial synthesis is the Haber-Bosch process. Arguably the most impactful of all chemical reactions on the modern world, its importance has been recognized by three Nobel prizes (Haber in 1918; Bosch in 1931; Ertl in 2007) and its ubiquity is such that half of the nitrogen atoms in our bodies have touched the iron catalysts of a Haber-Bosch reactor.
However, the Haber-Bosch process is not innocuous. Although the reaction is exothermic, the high activation energy required to break the nitrogen triple bond forms a kinetic barrier. As such, appreciable formation of ammonia is only obtained by running the synthesis at 400-500 oC, where the catalyst is most active, and 100-150 bar, to compensate for the equilibrium shift caused by the heat. Coupled with the massive scale of the reaction, these harsh conditions make Haber-Bosch one of the most energy-consuming industrial processes in the world; ammonia production uses up 2% of global energy and accounts for 3% of total CO2 emissions.2
It is no surprise that intensive research efforts have been geared towards achieving ammonia production under ambient conditions. The primary benefit is of course a reduction in energy consumption associated with heat. However, the industrial profile of ammonia production would also change with a room temperature synthesis. Currently, Haber-Bosch plants need to be large in order to offset their high production costs and turn a profit. With a low-cost, low-energy approach, production could become far more modular, with small-scale reactors being placed at the point of use. This decentralization would not only reduce transportation costs but also allow for fertilizer production in regions where large-scale Haber-Bosch infrastructure is lacking.
To achieve ambient ammonia production, a variety of reaction methodologies have been envisioned. Although approaches such as molecular catalysis and electrochemistry are interesting in their own right, the focus of the present post is on recent milestones attained through mechanochemistry. As the name implies, mechanochemistry harnesses mechanical force to drive chemical reactions forward. At the research scale, this is typically done in milling jars where balls are used to grind solid reagents together. Not only do these syntheses bypass the use (and waste) of solvents, but they can also often afford increased reaction yields and even access to previously unattainable products.
A significant breakthrough was achieved in 2021 when Han et al. reported a two-step mechanochemical synthesis of ammonia using commercially available iron powder as the catalyst.3 In this protocol, nitrogen dissociation first occurs on mechanically induced defects on the catalyst surface to yield iron nitrides. The ball mill is then emptied of nitrogen and refilled with hydrogen, and subsequent reduction of the iron nitride intermediates affords ammonia. The overall procedure requires about 2 days of milling, does not surpass 45 oC, and results in an ammonia concentration of 82.5 vol%, which is considerably higher than the 25 vol% of the conventional Haber-Bosch process.
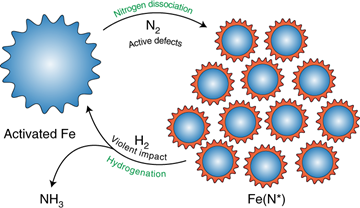
Still, Han et al.’s approach requires two discrete steps, which is not conducive to industrial application. Ideally, ammonia should be produced continuously without needing to change the gas supply. This important requirement was addressed in a recent work by Schüth and coworkers, who modified a ball mill to enable continuous ammonia synthesis in the presence of a cesium-promoted iron catalyst.4 Their most performant system was able to sustain the reaction for several days at room temperature. While certain details, such as catalyst activity at extended milling time and the need for moderate pressures (20 bar), can still be improved, this represents an impressive step forward for the realization of industrially relevant room-temperature ammonia production.


An interesting aspect of Schüth’s process pertains to the addition of promoters to the iron catalyst. Alkali promoters can improve the chemisorption ability of the catalyst, which greatly increases the amount of nitrogen that can be dissociated in the first step of the synthesis (N2 needs to first be adsorbed onto the catalyst before activation can occur). These promoters are common in traditional Haber-Bosch but are typically introduced as alkali oxides due to the volatility of the metals. However, the presence of oxygen tends to block nitrogen adsorption sites, which mitigates the benefits of the alkali promoter.5 In the mechanochemical approach, the use of mild conditions means that volatility is not an issue. As a result, cesium metal can be directly added to the iron catalyst (at around 2%), which greatly enhances catalytic activity. This example highlights how sustainable approaches to chemistry open up new possibilities due to their diverse ways of providing mass or energy transport.
Clearly, the future of sustainable fertilizers appears promising, but this may not be all. A recent perspective from MacFarlane and coauthors argues that beyond merely feeding the world, ammonia can be the highly practical energy carrier that is central to a so-called ammonia economy.6 To transition towards said economy, a multi-stage plan is proposed which starts from carbon capture at current Haber-Bosch plants and ultimately ends with a global shift to electrochemical ammonia synthesis. If the studies discussed above are of any indication, though, the final stage may well make use of the incessant grind of mechanochemistry. In the end, whether our ammonia comes from a ball mill or an electrode, the bottom line does not change: green methodologies are poised to not only feed our world but also galvanize it.
References
(1) How many people does synthetic fertilizer feed? https://ourworldindata.org/how-many-people-does-synthetic-fertilizer-feed (accessed 2022 -01 -17).
(2) Hattori, M.; Iijima, S.; Nakao, T.; Hosono, H.; Hara, M. Solid Solution for Catalytic Ammonia Synthesis from Nitrogen and Hydrogen Gases at 50 °C. Nat Commun 2020, 11 (1), 1–8. https://doi.org/10.1038/s41467-020-15868-8.
(3) Han, G.-F.; Li, F.; Chen, Z.-W.; Coppex, C.; Kim, S.-J.; Noh, H.-J.; Fu, Z.; Lu, Y.; Singh, C. V.; Siahrostami, S.; Jiang, Q.; Baek, J.-B. Mechanochemistry for Ammonia Synthesis under Mild Conditions. Nat. Nanotechnol. 2021, 16 (3), 325–330. https://doi.org/10.1038/s41565-020-00809-9.
(4) Reichle, S.; Felderhoff, M.; Schüth, F. Mechanocatalytic Room-Temperature Synthesis of Ammonia from Its Elements Down to Atmospheric Pressure. Angewandte Chemie International Edition 2021, 60 (50), 26385–26389. https://doi.org/10.1002/anie.202112095.
(5) Paál, Z.; Ertl, G.; Lee, S. B. Interactions of Potassium, Oxygen and Nitrogen with Polycrystalline Iron Surfaces. Applications of Surface Science 1981, 8 (3), 231–249. https://doi.org/10.1016/0378-5963(81)90119-7.
(6) MacFarlane, D. R.; Cherepanov, P. V.; Choi, J.; Suryanto, B. H. R.; Hodgetts, R. Y.; Bakker, J. M.; Ferrero Vallana, F. M.; Simonov, A. N. A Roadmap to the Ammonia Economy. Joule 2020, 4 (6), 1186–1205. https://doi.org/10.1016/j.joule.2020.04.004.