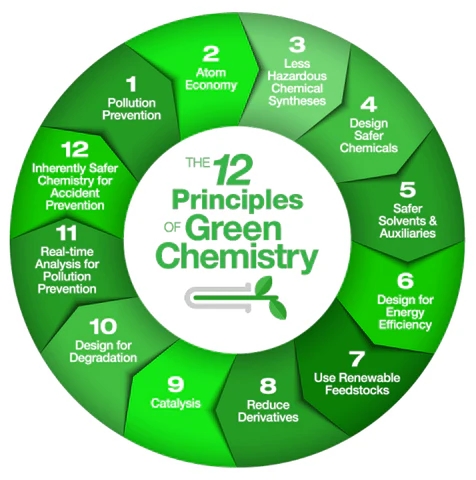
By Eloi Grignon, Ph.D. student, Member-at-Large for the GCI
Since their commercialization in 1991, lithium-ion batteries (LIBs) have gradually come to pervade our daily lives. Their ubiquity is achieved through our phones and laptops (you are likely reading these words via energy supplied by a LIB), where they are used to power not only our communication with one another, but also the myriad other tasks that we have come to delegate to our devices. Increasingly, LIBs are powering how we move, too, as is evidenced by the several million battery electric vehicles already on the road.1 With the production of electric vehicles set to skyrocket – the British and French governments have already pledged to ban sales of fossil-powered vehicles by 2040 – and the possibility of using LIBs for storage of grid electricity, it is clear that LIBs are not going anywhere, either.2 Indeed, spent batteries are expected to be generated at a rate of 2 million metric tons per year by 2030.3
And yet, there is no clear idea of what is to happen to these batteries once they’ve served their purpose. Currently, fewer than 5% of LIBs in the US and Europe are recycled while the rest end up in landfills.3
Since a LIB is densely comprised of several costly metals (Figure 1), it is fair to liken used batteries to enriched ore.3 It follows, then, that complete disposal of millions of metric tons of such a material represents a tremendous waste.
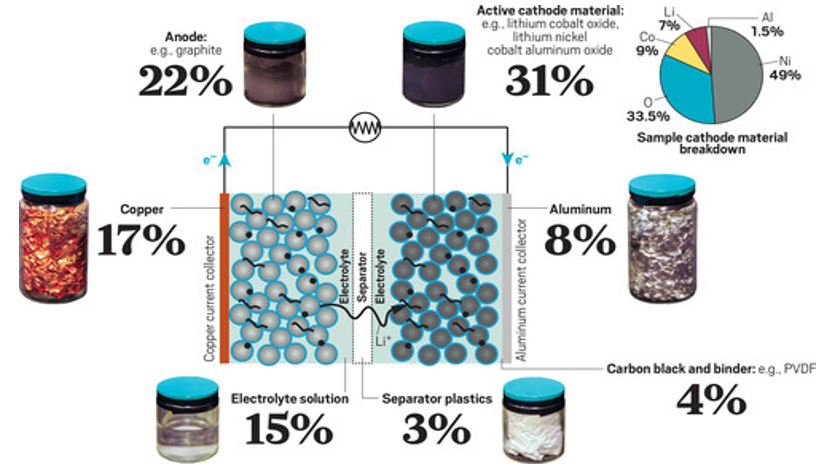
Figure 1. Breakdown of LIB constituents. From Ref [3].
Recycling could curb the waste by salvaging this ‘ore’ and supplying it to LIB manufacturers at a cheaper price than that of virgin materials, thereby reducing LIB cost. Moreover, less of the material would have to be mined and treated in the first place. This is especially important when considering the impacts of both processes: mining has obvious environmental consequences while ore treatment is typically energy-intensive and can release harmful gases such as SO
x.
4 Furthermore, 10-25% of global cobalt production is mined by ‘artisanal’ workers in the DRC, many of whom lack proper wages and equipment. The UNICEF estimated in 2012 that 40,000 children were employed in such mines.
5 From this perspective, LIB landfilling has a heavy economic, environmental, and moral opportunity cost attached.
In addition to wastefulness, landfilling LIBs also has direct negative consequences. Over time, the toxic constituents of the LIBs tend to flow into the soil, eventually leaching into the groundwater and accumulating in various organisms. These toxins can make their way up to humans, thus extending the health hazard to people. The harm imposed by discarded LIBs on the environment is not without some degree of irony, as LIBs have long been celebrated as a key cog in the establishment of a greener future. Evidently, this detrimental end-of-life scenario presents an incongruity.
Given the benefits of LIB recycling, it is clear that technical and economic barriers, rather than lack of purpose, are responsible for the poor recycling rates.
One such barrier results from the complicated composition within LIBs, which renders separation and recovery of all components difficult. For instance, smelting can effectively recover the heavy metals nickel, cobalt, and copper but fails to salvage lithium and the electrolyte. While hydrometallurgical (chemical leaching) methods can recover more components, they necessitate acids, hydrogen peroxide, and 7 m3 of water per ton of LIB.6 Needless to say, this is not ideal.
Another technical issue is the great variability between LIBs – different manufacturers tend to use different components and so there is no one universal composition (this pertains mainly to vehicles). As such, a recycling firm is at the mercy of its feedstock – for an input collected from many sources, there is no guarantee that 1 ton of batteries will yield a given amount of, say, cobalt.
These issues appear blatant when considering as a counterexample the success story of lead-acid battery recycling, whose simple and standardized composition – about 60% lead – enables an easy recycling process that claims nearly all (99%!) of used batteries.4
The low recycling rates are also due to economic factors. The end-to-end recycling process is energy-intensive and requires many steps, thus increasing costs. A firm operating such a process must carefully assess whether their repurposing protocol is cheap enough to supply materials that are price-competitive with mined materials. Due to the lack of LIB standardization and high volatility of constituent prices, this assessment is far from trivial and the business represents a clear risk. This risk is further exacerbated by the uncertainty of what the future of energy storage may resemble. In the arms race for higher energy density, new technologies arise frequently, thus threatening to render state-of-the-art materials (and so, recycling processes) obsolete.
While the above paragraphs appear rather pessimistic, it should be noted that we are only at the onset of the LIB boom. Indeed, the field of LIB recycling is still gaining traction and it is expected that serious investments will aid in the development of more efficient recycling techniques. To this end, the US DoE (through the $15 million ReCell Center) and the UK-based ReLib project have pledged to fund and support R&D in LIB recycling.3
There is also a clear interest from the private sector as is evidenced by the numerous startup firms currently designing their own protocols, including the Toronto-based company Li-Cycle.7
Another approach to sustainable energy storage is to circumvent the need for recycling breakthroughs altogether by designing the LIB differently from the start. For instance, the use of organic materials that are easily recyclable is increasingly explored for use in devices.1 Not only are these materials favourable in the end-of-life stage, but their production is also cheap, accessible, and environmentally benign.
In any case, scientists, engineers, and policymakers must come together to address the issues caused by LIB landfilling. And quickly, too, because the storm is coming (Figure 2).
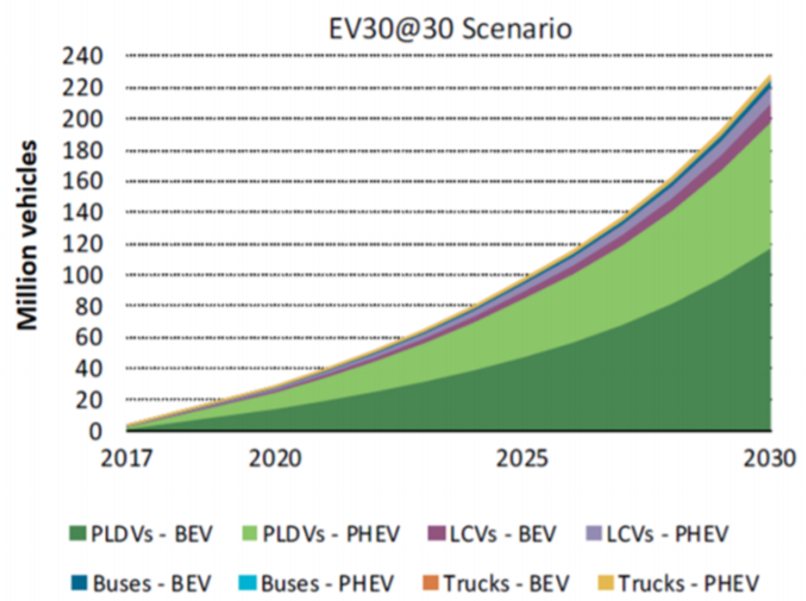
Figure 2. A possible scenario for the growth of electric vehicle sales in the next decade. PLDVs = passenger light duty vehicles; LCVs = light commercial vehicles; BEVs = battery electric vehicles; PHEV = plug-in hybrid electric vehicles. From Ref [1].
- Poizot, P.; Gaubicher, J.; Renault, S.; Dubois, L.; Liang, Y.; Yao, Y. J. C. R., Opportunities and Challenges for Organic Electrodes in Electrochemical Energy Storage. 2020.
- Gardiner, J. J. T. G., The rise of electric cars could leave us with a big battery waste problem. 2017, 10.
- It’s time to recycle lithium-ion batteries. C&EN Global Enterprise 2019, 97 (28), 29-32.
- Gaines, L. J. S. M.; Technologies, The future of automotive lithium-ion battery recycling: Charting a sustainable course. 2014, 1, 2-7.
- Frankel, T. C.; Chavez, M. R.; Ribas, J. J. T. W. P., The cobalt pipeline. Tracing the path from deadly hand-dug mines in Congo to consumers’ phones and laptops. 2016, 30.
- Larcher, D.; Tarascon, J.-M. J. N. c., Towards greener and more sustainable batteries for electrical energy storage. 2015, 7 (1), 19-29.
- https://li-cycle.com/about-us/.